Lithium-ion battery
Nokia Li-ion battery for powering a mobile phone |
|
| specific energy | (0.36-0.90 MJ/kg) |
|---|---|
| energy density | (0.90-2.23 MJ/L) |
| specific power | ~250-~340 W/kg[1] |
| Charge/discharge efficiency | 80-90%[3] |
| Energy/consumer-price | 2.5 W·h/US$[4] |
| Self-discharge rate | 8% at 21 °C 15% at 40 °C 31% at 60 °C (per month)[5] |
| Cycle durability |
400-1200 cycles [6] |
| Nominal cell voltage | 3.6 / 3.7 V |
A lithium-ion battery (sometimes Li-ion battery or LIB) is a family of rechargeable battery types in which lithium ions move from the negative electrode to the positive electrode during discharge, and back when charging. Chemistry, performance, cost, and safety characteristics vary across LIB types. Unlike lithium primary batteries (which are disposable), lithium-ion electrochemical cells use an intercalated lithium compound as the electrode material instead of metallic lithium.
Lithium-ion batteries are common in consumer electronics. They are one of the most popular types of rechargeable battery for portable electronics, with one of the best energy densities, no memory effect, and a slow loss of charge when not in use. Beyond consumer electronics, LIBs are also growing in popularity for military, electric vehicle, and aerospace applications.[7] Research is yielding a stream of improvements to traditional LIB technology, focusing on energy density, durability, cost, and intrinsic safety.
Charge and discharge
During discharge, lithium ions Li+ carry the current from the negative to the positive electrode, through the non-aqueous electrolyte and separator diaphragm.[8]
During charging, an external electrical power source (the charging circuit) applies a higher voltage (but of the same polarity) than that produced by the battery, forcing the current to pass in the reverse direction. The lithium ions then migrate from the positive to the negative electrode, where they become embedded in the porous electrode material in a process known as intercalation.
Construction
The three primary functional components of a lithium-ion battery are the anode, cathode, and electrolyte. The anode of a conventional lithium-ion cell is made from carbon, the cathode is a metal oxide, and the electrolyte is a lithium salt in an organic solvent.[9]
The most commercially popular anode material is graphite. The cathode is generally one of three materials: a layered oxide (such as lithium cobalt oxide), a polyanion (such as lithium iron phosphate), or a spinel (such as lithium manganese oxide).[10]
The electrolyte is typically a mixture of organic carbonates such as ethylene carbonate or diethyl carbonate containing complexes of lithium ions.[11] These non-aqueous electrolytes generally use non-coordinating anion salts such as lithium hexafluorophosphate (LiPF6), lithium hexafluoroarsenate monohydrate (LiAsF6), lithium perchlorate (LiClO4), lithium tetrafluoroborate (LiBF4), and lithium triflate (LiCF3SO3).
Depending on materials choices, the voltage, capacity, life, and safety of a lithium-ion battery can change dramatically. Recently, novel architectures using nanotechnology have been employed to improve performance.
Pure lithium is very reactive. It reacts vigorously with water to form lithium hydroxide and hydrogen gas. Thus, a non-aqueous electrolyte is typically used, and a sealed container rigidly excludes water from the battery pack.
Lithium ion batteries are more expensive than NiCd batteries but operate over a wider temperature range with higher energy densities, while being smaller and lighter. They are fragile and so need a protective circuit to limit peak voltages.
Formats
Li-ion cells are available in various formats, which can generally be divided into four groups:[12][13]
- Small cylindrical (solid body without terminals, such as those used in laptop batteries)
- Large cylindrical (solid body with large threaded terminals)
- Pouch (soft, flat body, such as those used in cell phones)
- Prismatic (semi-hard plastic case with large threaded terminals, often used in vehicles' traction packs)
The lack of case gives pouch cells the highest energy density; however, pouch cells (and prismatic cells) require an external means of containment to prevent expansion when their state-of-charge (SOC) level is high.[14]
History
Lithium batteries were first proposed by M.S. Whittingham, now at Binghamton University, while working for Exxon in the 1970s.[15] Whittingham used titanium(II) sulfide as the cathode and lithium metal as the anode.
The reversible intercalation in graphite[16][17] and intercalation into cathodic oxides [18][19] was also already discovered in the 1970s by J.O. Besenhard at TU Munich. He also proposed the application as high energy density lithium cells[20][21]
Primary lithium batteries in which the anode is made from metallic lithium pose safety issues. As a result, lithium-ion batteries were developed in which both anode and cathode are made of a material containing lithium ions.
In 1979, John Goodenough demonstrated a rechargeable cell with high cell voltage in the 4V range using lithium cobalt oxide (LiCoO2) as the positive electrode and lithium metal as the negative electrode.[22] This innovation provided the positive electrode material which made LIBs possible. LiCoO2 is a stable positive electrode material which acts as a donor of lithium ions, which means that it can be used with a negative electrode material other than lithium metal. By enabling the use of stable and easy-to-handle negative electrode materials, LiCoO2 opened a whole new range of possibilities for novel rechargeable battery systems.
In 1981, Bell Labs developed a workable graphite anode[23] to provide an alternative to the lithium metal battery.
In 1982, Rachid Yazami demonstrated the reversible electrochemical intercalation of lithium in graphite.[24][25] The organic electrolytes available at the time would decompose during charging if used with a graphite negative electrode, preventing the early development of a rechargeable battery which employed the lithium/graphite system. Yazami used a solid electrolyte to demonstrate that lithium could be reversibly intercalated in graphite through an electrochemical mechanism.
In 1983, Dr. Michael Thackeray, Goodenough, and coworkers identified manganese spinel as a cathode material.[26] Spinel showed great promise, given its low-cost, good electronic and lithium ion conductivity, and three-dimensional structure, which gives it good structural stability. Although pure manganese spinel fades with cycling, this can be overcome with chemical modification of the material.[27] Manganese spinel is currently used in commercial cells.[28]
In 1985, Akira Yoshino assembled a prototype cell using carbonaceous material into which lithium ions could be inserted as the anode, and as the cathode lithium cobalt oxide (LiCoO2), which is stable in air.[29] By using an anode material without metallic lithium, safety was dramatically improved over batteries which used lithium metal. The use of lithium cobalt oxide (LiCoO2) enabled industrial-scale production to be achieved easily.
This was the birth of the current lithium-ion battery.
Modern batteries
In 1991, Sony and Asahi Kasei released the first commercial lithium-ion battery.
In 1989, Goodenough and Arumugam Manthiram of the University of Texas at Austin showed that cathodes containing polyanions, e.g., sulfates, produce higher voltages than oxides due to the inductive effect of the polyanion.[30]
In 1996, Goodenough, Akshaya Padhi and coworkers identified lithium iron phosphate (LiFePO4) and other phospho-olivines (lithium metal phosphates with the same structure as mineral olivine) as cathode materials.[31]
In 2002, Yet-Ming Chiang and his group at MIT showed a substantial improvement in the performance of lithium batteries by boosting the material's conductivity by doping it with aluminium, niobium and zirconium. The exact mechanism causing the increase became the subject of widespread debate.[32]
In 2004, Chiang again increased performance by utilizing iron phosphate particles of less than 100 nanometers in diameter. This decreased particle density almost one hundredfold, increased the cathode's surface area and improved capacity and performance. Commercialization led to a rapid growth in the market for higher capacity LIBs, as well as a patent infringement battle between Chiang and Goodenough.[32]
As of 2011, lithium-ion batteries account for 67% of all portable secondary battery sales in Japan.[33]
Electrochemistry
The three participants in the electrochemical reactions in a lithium-ion battery are the anode, cathode, and electrolyte.
Both the anode and cathode are materials into which, and from which, lithium can migrate. During insertion (or intercalation) lithium moves into the electrode. During the reverse process, extraction (or deintercalation), lithium moves back out. When a lithium-based cell is discharging, the lithium is extracted from the anode and inserted into the cathode. When the cell is charging, the reverse occurs.
Useful work can only be extracted if electrons flow through a closed external circuit. The following equations are in units of moles, making it possible to use the coefficient 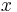 .
.
The positive electrode half-reaction (with charging being forwards) is: [34]
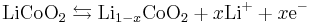
The negative electrode half-reaction is:
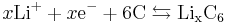
The overall reaction has its limits. Overdischarge supersaturates lithium cobalt oxide, leading to the production of lithium oxide,[35] possibly by the following irreversible reaction:
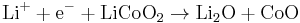
Overcharge up to 5.2 Volts leads to the synthesis of cobalt(IV) oxide, as evidenced by x-ray diffraction[36]
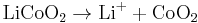
In a lithium-ion battery the lithium ions are transported to and from the cathode or anode, with the transition metal, cobalt (Co), in 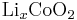 being oxidized from Co3+ to Co4+ during charging, and reduced from Co4+ to Co3+ during discharge.
being oxidized from Co3+ to Co4+ during charging, and reduced from Co4+ to Co3+ during discharge.
Positive electrodes
| Electrode material | Average potential difference | Specific capacity | Specific energy |
|---|---|---|---|
| LiCoO2 | 3.7 V | 140 mA·h/g | 0.518 kW·h/kg |
| LiMn2O4 | 4.0 V | 100 mA·h/g | 0.400 kW·h/kg |
| LiNiO2 | 3.5 V | 180 mA·h/g | 0.630 kW·h/kg |
| LiFePO4 | 3.3 V | 150 mA·h/g | 0.495 kW·h/kg |
| Li2FePO4F | 3.6 V | 115 mA·h/g | 0.414 kW·h/kg |
| LiCo1/3Ni1/3Mn1/3O2 | 3.6 V | 160 mA·h/g | 0.576 kW·h/kg |
| Li(LiaNixMnyCoz)O2 | 4.2 V | 220 mA·h/g | 0.920 kW·h/kg |
Negative electrodes
| Electrode material | Average potential difference | Specific capacity | Specific energy |
|---|---|---|---|
| Graphite (LiC6) | 0.1-0.2 V | 372 mA·h/g | 0.0372-0.0744 kW·h/kg |
| Hard Carbon (LiC6) | ? V | ? mA·h/g | ? kW·h/kg |
| Titanate (Li4Ti5O12) | 1-2 V | 160 mA·h/g | 0.16-0.32 kW·h/kg |
| Si (Li4.4Si)[37] | 0.5-1 V | 4212 mA·h/g | 2.106-4.212 kW·h/kg |
| Ge (Li4.4Ge)[38] | 0.7-1.2 V | 1624 mA·h/g | 1.137-1.949 kW·h/kg |
Electrolytes
The cell voltages given in the Electrochemistry section are larger than the potential at which aqueous solutions can electrolyze, in addition lithium is highly reactive to water, therefore, nonaqueous or aprotic solutions are used.
Liquid electrolytes in lithium-ion batteries consist of lithium salts, such as LiPF6, LiBF4 or LiClO4 in an organic solvent, such as ethylene carbonate, dimethyl carbonate, and diethyl carbonate. A liquid electrolyte conducts lithium ions, acting as a carrier between the cathode and the anode when a battery passes an electric current through an external circuit. Typical conductivities of liquid electrolyte at room temperature (20 °C (68 °F)) are in the range of 10 mS/cm (1 S/m), increasing by approximately 30–40% at 40 °C (104 °F) and decreasing by a slightly smaller amount at 0 °C (32 °F)[39]
Unfortunately, organic solvents easily decompose on anodes during charging. However, when appropriate organic solvents are used as the electrolyte, the solvent decomposes on initial charging and forms a solid layer called the solid electrolyte interphase (SEI),[40] which is electrically insulating yet provides sufficient ionic conductivity. The interphase prevents decomposition of the electrolyte after the second charge. For example, ethylene carbonate is decomposed at a relatively high voltage, 0.7 V vs. lithium, and forms a dense and stable interface.
A good solution for the interface instability is the application of a new class of composite electrolytes based on POE (poly(oxyethylene)) developed by Syzdek et al.[41][42] It can be either solid (high molecular weight) and be applied in dry Li-polymer cells, or liquid (low molecular weight) and be applied in regular Li-ion cells.
Another issue that Li-ion technology is facing is safety. Large scale application of Li cells in Electric Vehicles needs a dramatic decrease in the failure rate. One of the solutions is the novel technology based on reversed-phase composite electrolytes, employing porous ceramic material filled with electrolyte.[43]
Advantages and disadvantages
Note that both advantages and disadvantages depend on the materials and design that make up the battery. This summary reflects older designs that use carbon anode, metal oxide cathodes, and lithium salt in an organic solvent for the electrolyte.
Advantages
- Wide variety of shapes and sizes efficiently fitting the devices they power.
- Much lighter than other energy-equivalent secondary batteries.[44]
- High open circuit voltage in comparison to aqueous batteries (such as lead acid, nickel-metal hydride and nickel-cadmium).[45] This is beneficial because it increases the amount of power that can be transferred at a lower current.
- No memory effect.
- Self-discharge rate of approximately 5-10% per month, compared to over 30% per month in common nickel metal hydride batteries, approximately 1.25% per month for Low Self-Discharge NiMH batteries and 10% per month in nickel-cadmium batteries.[46] According to one manufacturer, lithium-ion cells (and, accordingly, "dumb" lithium-ion batteries) do not have any self-discharge in the usual meaning of this word.[34] What looks like a self-discharge in these batteries is a permanent loss of capacity (see Disadvantages). On the other hand, "smart" lithium-ion batteries do self-discharge, due to the drain of the built-in voltage monitoring circuit.
- Components are environmentally safe as there is no free lithium metal.
Disadvantages
Cell life
- Charging forms deposits inside the electrolyte that inhibit ion transport. Over time, the cell's capacity diminishes. The increase in internal resistance reduces the cell's ability to deliver current. This problem is more pronounced in high-current applications. The decrease means that older batteries do not charge as much as new ones (charging time required decreases proportionally).
- High charge levels and elevated temperatures (whether from charging or ambient air) hasten capacity loss.[47] Charging heat is caused by the carbon anode (typically replaced with lithium titanate which drastically reduces damage from charging, including expansion and other factors).[48]
Internal resistance
- The internal resistance of standard (Cobalt) lithium-ion batteries is high compared to both other rechargeable chemistries such as nickel-metal hydride and nickel-cadmium, and LiFePO4 and lithium-polymer cells.[49] Internal resistance increases with both cycling and age.[50][51][52] Rising internal resistance causes the voltage at the terminals to drop under load, which reduces the maximum current draw. Eventually increasing resistance means that the battery can no longer operate for an adequate period.
- To power larger devices, such as electric cars, connecting many small batteries in a parallel circuit is more effective[53] and efficient than connecting a single large battery.[54]
Safety requirements
If overheated or overcharged, Li-ion batteries may suffer thermal runaway and cell rupture.[55] In extreme cases this can lead to combustion. Deep discharge may short-circuit the cell, in which case recharging would be unsafe.[56] To reduce these risks, Lithium-ion battery packs contain fail-safe circuitry that shuts down the battery when its voltage is outside the safe range of 3–4.2 V per cell.[34][46] When stored for long periods the small current draw of the protection circuitry itself may drain the battery below its shut down voltage; normal chargers are then ineffective. Many types of lithium-ion cell cannot be charged safely below 0°C.[57]
Other safety features are required in each cell:[34]
- Shut-down separator (for overtemperature)
- Tear-away tab (for internal pressure)
- Vent (pressure relief)
- Thermal interrupt (overcurrent/overcharging)
These devices occupy useful space inside the cells, add additional points of failure and irreversibly disable the cell when activated. They are required because the anode produces heat during use, while the cathode may produce oxygen. These devices and improved electrode designs reduce/eliminate the risk of fire or explosion.
These safety features increase costs compared to nickel metal hydride batteries, which require only a hydrogen/oxygen recombination device (preventing damage due to mild overcharging) and a back-up pressure valve.[46]
Specifications and design
- Specific energy density: 150 to 250 W·h/kg (540 to 900 kJ/kg)[1]
- Volumetric energy density: 250 to 620 W·h/l (900 to 1900 J/cm³)[2]
- Specific power density: 300 to 1500 W/kg (@ 20 seconds and 285 W·h/l)[1]
Because lithium-ion batteries can have a variety of cathode and anode materials, the energy density and voltage vary accordingly.
Lithium-ion batteries with a lithium iron phosphate cathode and graphite anode have a nominal open-circuit voltage of 3.2 V and a typical charging voltage of 3.6 V. Lithium nickel manganese cobalt (NMC) oxide cathode with graphite anodes have a 3.7 V nominal voltage with a 4.2 V max charge. The charging procedure is performed at constant voltage with current-limiting circuitry (i.e., charging with constant current until a voltage of 4.2 V is reached in the cell and continuing with a constant voltage applied until the current drops close to zero). Typically, the charge is terminated at 3% of the initial charge current. In the past, lithium-ion batteries could not be fast-charged and needed at least two hours to fully charge. Current-generation cells can be fully charged in 45 minutes or less. Some lithium-ion varieties can reach 90% in as little as 10 minutes.[58]
Battery charging procedure
The charging procedures for single Li-ion cells, and complete Li-ion batteries, are slightly different.
- A single Li-ion cell is charged in 2 stages:
- CC
- CV
- CC
- Balance (not required once a battery is balanced)
- CV
Stage 1: CC: Apply charging current to the battery, until the voltage limit per cell is reached.
Stage 2: Balance: Reduce the charging current (or cycle the charging on and off to reduce the average current) while the State Of Charge of individual cells is balanced by a balancing circuit, until the battery is balanced.
Stage 3: CV: Apply a voltage equal to the maximum cell voltage times the number of cells in series to the battery, as the current gradually declines asymptotically towards 0, until the current is below a set threshold
Variations in materials and construction
The increasing demand for batteries has led vendors and academics to focus on improving the power density, operating temperature, safety, durability, charging time, output power, and cost of LIB solutions.
| Area | Technology | Researchers | Target application | Date | Benefit |
|---|---|---|---|---|---|
| Cathode | Manganese spinel (LMO) | Lucky Goldstar Chemical,[61] NEC, Samsung,[62] Hitachi,[63] Nissan/AESC[64] | Hybrid electric vehicle, cell phone, laptop | 1996 | durability, cost |
| Lithium iron phosphate | University of Texas/Hydro-Québec,[65]/Phostech Lithium Inc., Valence Technology, A123Systems/MIT[66][67] | Segway Personal Transporter, power tools, aviation products, automotive hybrid systems, PHEV conversions | 1996 | moderate density (2 A·h outputs 70 amperes) operating temperature >60 °C (140 °F) | |
| Lithium nickel manganese cobalt (NMC) | Imara Corporation, Nissan Motor[68][69] | 2008 | density, output, safety | ||
| LMO/NMC | Sony, Sanyo | power, safety (although limited durability) | |||
| Lithium iron fluorophosphate | University of Waterloo[70] | 2007 | durability, cost (replace Li with Na or Na/Li) | ||
| Lithium air | University of Dayton Research Institute[71] | automotive | 2009 | density, safety[71] | |
| 5% Vanadium-doped Lithium iron phosphate olivine | Binghamton University[72] | 2008 | output | ||
| Anode | Lithium-titanate battery (LT) | Altairnano | automotive (Phoenix Motorcars), electrical grid (PJM Interconnection Regional Transmission Organization control area,[73] United States Department of Defense[74]), bus (Proterra[75]) | 2008 | output, charging time, durability (20 years, 9,000 cycles), safety, operating temperature (-50–70 °C (-58–158 °F)[76] |
| Lithium vanadium oxide | Samsung/Subaru.[77] | automotive | 2007 | density (745Wh/l)[78] | |
| Cobalt-oxide nanowires from genetically modified virus | MIT | 2006 | density, thickness[79] | ||
| Three-Dimensional (3D) Porous Particles Composed of Curved Two-Dimensional (2D) Nano-Sized Layers | Georgia Institute of Technology [80] | high energy batteries for electronics and electrical vehicles | 2011 | specific capacity > 2000 mA·h/g, high efficiency, rapid low-cost synthesis [81] | |
| Iron-phosphate nanowires from genetically modified virus | MIT | 2009 | density, thickness[82][83][84] | ||
| Silicon/titanium dioxide composite nanowires from genetically modified tobacco virus | University of Maryland | explosive detection sensors, biomimetic structures, water-repellent surfaces, micro/nano scale heat pipes | 2010 | density, low charge time[85] | |
| Porous silicon/carbon nanocomposite spheres | Georgia Institute of Technology | portable electronics, electrical vehicles, electrical grid | 2010 | high stability, high capacity, low charge time[86] | |
| nano-sized wires on stainless steel | Stanford University | wireless sensors networks, | 2007 | density[87][88] (shift from anode- to cathode-limited), durability issue remains (wire cracking) | |
| Metal hydrides | Laboratoire de Réactivité et de Chimie des Solides, General Motors | 2008 | density (1480 mA·h/g)[89] | ||
| Silicon Nanotubes (or Silicon Nanospheres) Confined within Rigid Carbon Outer Shells | Georgia Institute of Technology, MSE, NanoTech Yushin's group[90] | stable high energy batteries for cell phones, laptops, netbooks, radios, sensors and electrical vehicles | 2010 | specific capacity 2400 mA·h/g, ultra-high Coulombic Efficiency and outstanding SEI stability [91] | |
| Silicon nano-powder in a conductive polymer binder | Lawrence Berkeley National Laboratory, Environmental Energy Technologies Division [92] | Automotive and Electronics | 2011 | high capacity anodes (1400 mA·h/g) with good cycling characteristics | |
| Electrode | LT/LMO | Ener1/Delphi,[93][94] | 2006 | durability, safety (limited density) | |
| Nanostructure | Université Paul Sabatier/Université Picardie Jules Verne[95] | 2006 | density |
Usage guidelines
Prolonging battery pack life
- Depletion below the low-voltage threshold (2.4 to 2.8 V/cell, depending on chemistry) results in a dead battery which does not even appear to charge because the protection circuit (a type of electronic fuse) disables it.[96]
- Lithium-ion batteries should be kept cool; they may be stored in a refrigerator.[96][97]
- The rate of degradation of Lithium-ion batteries is strongly temperature-dependent; they degrade much faster if stored or used at higher temperatures.[96][98]
Multicell devices
Li-ion batteries require a battery management system to prevent operation outside each cell's safe operating area (over-charge, under-charge, safe temperature range) and to balance cells to eliminate SOC mismatches, significantly improving battery efficiency and increasing overall capacity.[99] As the number of cells and load currents increase, the potential for mismatch also increases.[100] There are two kinds of mismatch in the pack: state-of-charge (SOC) and capacity/energy ("C/E") mismatch. Though SOC is more common, each problem limits pack capacity (mA·h) to the capacity of the weakest cell.
Safety
Lithium-ion batteries can rupture, ignite, or explode when exposed to high temperature. Short-circuiting a battery will cause the cell to overheat and possibly to catch fire. Adjacent cells may then overheat and fail, possibly causing the entire battery to ignite or rupture. In the event of a fire, the device may emit dense irritating smoke.[101]
Replacing the lithium cobalt oxide cathode material in lithium-ion batteries with a lithium metal phosphate such as lithium iron phosphate, improves cycle counts, shelf life and safety, but lowers capacity. Currently, these 'safer' lithium-ion batteries are mainly used in electric cars and other large-capacity battery applications, where safety issues are critical.[102]
Lithium-ion batteries normally contain safety devices to protect the cells from disturbance. However, contaminants inside the cells can defeat these safety devices.
Recalls
In March 2007, Lenovo recalled approximately 205,000 batteries at risk of explosion. In August 2007, Nokia recalled over 46 million batteries at risk of overheating and exploding.[103] One such incident occurred in the Philippines involving a Nokia N91, which uses the BL-5C battery.[104]
In December 2006, Dell recalled approximately 22,000 laptop batteries from the US market.[105] Approximately 10 million Sony batteries used in Dell, Sony, Apple, Lenovo/IBM, Panasonic, Toshiba, Hitachi, Fujitsu and Sharp laptops were recalled in 2006. The batteries were found to be susceptible to internal contamination by metal particles. Under some circumstances, these particles could pierce the separator, causing a short-circuit.[106]
In October 2004, Kyocera Wireless recalled approximately 1 million mobile phone batteries to identify counterfeits.[107]
Transport restrictions
In January 2008, the United States Department of Transportation ruled that passengers on commercial aircraft could carry lithium batteries in their checked baggage if the batteries are installed in a device. Types of batteries affected by this rule are those containing lithium, including Li-ion, lithium polymer, and lithium cobalt oxide chemistries. Lithium-ion batteries containing more than 25 grams (0.88 oz) equivalent lithium content (ELC) are exempt from the rule and are forbidden in air travel.[108] This restriction greatly reduces the chances of the batteries short-circuiting and causing a fire.
Additionally, a limited number of replacement batteries may be transported in carry-on luggage. Such batteries must be sealed in their original protective packaging or in individual containers or plastic bags.[108][109]
Some postal administrations restrict air shipping (including EMS) of lithium and lithium-ion batteries, and products containing these (for example: laptops, cell phones). Among these countries and regions are Hong Kong,[110] Australia and Japan.[111]
Research
Researchers are working to improve the power density, safety, recharge cycle, cost and other characteristics of these batteries.
Solid-state designs [112] have the potential to deliver three times the energy density of typical 2011 lithium-ion batteries at less than half the cost per kilowatt-hour. This approach eliminates binders, separators, and liquid electrolytes. By eliminating these, "you can get around 95% of the theoretical energy density of the active materials." [113]
Earlier trials of this technology encountered cost barriers, because the semiconductor industry's vacuum deposition technology cost 20–30 times too much. The new process deposits semiconductor-quality films from a solution. The nanostructured films grow directly on a substrate and then sequentially on top of each other. The process allows the firm to "spray-paint a cathode, then a separator/electrolyte, then the anode. It can be cut and stacked in various form factors.[113]
See also
| Book: Lithium-ion batteries | |
| Wikipedia books are collections of articles that can be downloaded or ordered in print. | |
Notes
- ^ a b c d "Rechargeable Li-Ion OEM Battery Products". Panasonic.com. http://www.panasonic.com/industrial/batteries-oem/oem/lithium-ion.aspx. Retrieved 23 April 2010.
- ^ a b "Panasonic Develops New Higher-Capacity 18650 Li-Ion Cells; Application of Silicon-based Alloy in Anode". greencarcongress.com. http://www.greencarcongress.com/2009/12/panasonic-20091225.html. Retrieved 31 January 2011.
- ^ Valøen & Shoesmith (2007). The effect of PHEV and HEV duty cycles on battery and battery pack performance (PDF). 2007 Plug-in Highway Electric Vehicle Conference: Proceedings. Retrieved 11 June 2010.
- ^ 11.10V,6600Mah, Li-Ion, Replacement Laptop Battery For Dell (etc). Amazon.com Online Store. Retrieved 11 June 2010.
- ^ H. Abea, T. Muraia and K. Zaghibb (1999). Vapor-grown carbon fiber anode for cylindrical lithium ion rechargeable batteries. Journal of Power Sources 77:2, February 1999, pp. 110-115. DOI:10.1016/S0378-7753(98)00158-X. Retrieved 11 June 2010.
- ^ Battery Types and Characteristics for HEV ThermoAnalytics, Inc., 2007. Retrieved 11 June 2010.
- ^ Ballon, Massie Santos (14 October 2008). "Electrovaya, Tata Motors to make electric Indica". cleantech.com. Cleantech Group. http://www.cleantech.com/news/3694/electrovaya-tata-motors-make-electric-indica. Retrieved 11 June 2010.
- ^ David Linden, Thomas B. Reddy (ed). Handbook Of Batteries 3rd Edition. McGraw-Hill, New York, 2002 ISBN 0-07-135978-8 chapter 35
- ^ Silberberg, M. 2006. Chemistry: The Molecular Nature of Matter and Change, 4th Ed. New York (NY): McGraw-Hill Education. p 935.
- ^ Thackeray, Thomas, and Whittingham (March 2000). Science and Applications of Mixed Conductors for Lithium Batteries. mrs.com; Materials Research Society. Retrieved 11 June 2010.
- ^ MSDS: National Power Corp Lithium Ion Batteries (PDF). tek.com; Tektronix Inc., 7 May 2004. Retrieved 11 June 2010.
- ^ Battery Management Systems for Large Lithium-Ion Battery Packs page 2
- ^ "Cell boards for various cell formats". Elithion.com. http://elithion.com/lithiumate-pro-cell-boards.php. Retrieved 8 October 2011.
- ^ Battery Management Systems for Large Lithium-Ion Battery Packs page 234
- ^ M Stanley Whittingham. Electrical Energy Storage and Intercalation Chemistry Science: 192 (4244): 1126
- ^ J.O.Besenhard and H.P. Fritz, Cathodic Reduction of Graphite in Organic Solutions of Alkali and NR 4+ Salts, J. Electroanal. Chem., 53, 329 (1974)
- ^ J.O. Besenhard, The Electrochemical Preparation and Properties of Ionic Alkali Metal and NR 4+ Graphite Intercalation Compounds in Organic Electrolytes, Carbon, 14, 111 (1976)
- ^ R. Schallhorn, R. Kuhlmann, and J.O. Besenhard, Topotactic Redox Reactions and Ion Exchange of Layered MoO3 Bronzes,, Mat. Res. Bull., 11, 83 (1976)
- ^ J.O. Besenhard and R. Schallhorn, The Discharge Reaction Mechanism of the MoO3 Electrode in Organic Electrolytes” J. Power Sources, 1, 267 (1976/77)
- ^ J.O. Besenhard and G. Eichinger, High Energy Density Lithium Cells. Part I. Electrolytes and Anodes, J. Electroanal. Chem., 68, 1 (1976); and G. Eichinger
- ^ J.O. Besenhard, High Energy Density Lithium Cells. Part II. Cathodes and Complete Cells, J. Electroanal. Chem., 72, 1 (1976)
- ^ "USPTO search for inventions by "Goodenough, John"". Patft.uspto.gov. http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&u=%2Fnetahtml%2FPTO%2Fsearch-adv.htm&r=0&f=S&l=50&d=PTXT&RS=IN%2F%22goodenough%22&Refine=Refine+Search&Refine=Refine+Search&Query=in%2F%22goodenough%2C+john%22. Retrieved 8 October 2011.
- ^ US 4304825, Basu; Samar, "Rechargeable battery", issued 8 December 1981, assigned to Bell Telephone Laboratories
- ^ International Meeting on Lithium Batteries, Rome, 27–29 April 1982, C.L.U.P. Ed. Milan, Abstract #23
- ^ Journal of Power Sources (April–May 1983), 9 (3–4), 365–371
- ^ M.M. Thackeray, W.I.F. David, P.G. Bruce, and J.B. Goodenough (4 February 1983). "Lithium insertion into manganese spinels". Materials Research Bulletin (Elsevier) 18 (4): 461–472. doi:10.1016/0025-5408(83)90138-1.
- ^ Gholamabbas Nazri, Gianfranco Pistoia (2004). Lithium batteries: science and ... - Google Books. Springer. ISBN 9781402076282. http://books.google.com/?id=k4duxuea3eIC. Retrieved 8 October 2009.
- ^ Voelcker, John (September 2007). Lithium Batteries Take to the Road IEEE Spectrum.
- ^ US 4668595, Yoshino; Akira, "Secondary Battery", issued 10 May 1985, assigned to Asahi Kasei
- ^ A. Manthiram and J.B. Goodenough Corresponding (16 May 1989). "Lithium insertion into Fe2(SO4)3 frameworks". Journal of Power Sources (Elsevier B.V.) 26 (3–4): 403–408. doi:10.1016/0378-7753(89)80153-3.
- ^ Padhi, A. K. (1997). "Phospho-olivines as positive-electrode materials for rechargeable lithium batteries". Electrochem. Society 144 (4): 1188–1194. doi:10.1149/1.1837571. http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=JESOAN000144000004001188000001&idtype=cvips&gifs=yes.
- ^ a b Editors (6 March 2008). "In search of the perfect battery" (PDF). The Economist. Archived from the original on 25 September 2009. http://www.mitenergyclub.org/assets/2009/9/25/Economist_Batteries2008.pdf. Retrieved 11 May 2010.
- ^ [1] Monthly battery sales statistics - MoETI - March 2011
- ^ a b c d Staff (November 2003) (PDF). Lithium Ion technical handbook. Gold Peak Industries Ltd.. http://www.gpbatteries.com/html/pdf/Li-ion_handbook.pdf.
- ^ H.C. Choi et al., J. Phys. Chem. B 107 p5806(2003) doi:10.1021/jp030438w
- ^ G.G. Amatucci, J.M. Tarascon, L.C. Kein J. Electrochemical Society 143 p1114 1996 doi:10.1149/1.1836594
- ^ R. Ruffo; S. S. Hong, C. K. Chan, R. A. Huggins, Y. Cui (2009). "Impedance Analysis of Silicon Nanowire Lithium Ion Battery Anodes" (PDF). J. Phys. Chem. C. 113 (113 (26), (2009)): 11390–11398. doi:10.1021/jp901594g. http://www.stanford.edu/group/cui_group/papers/Impedance_jpc.pdf. Retrieved 1 September 2009.
- ^ C. K. Chan; X. F. Zhang, Y. Cui (2007). "High Capacity Li-ion Battery Anodes Using Ge Nanowires" (PDF). Nano Lett. 8 (8 (2007)): 307–309. Bibcode 2008NanoL...8..307C. doi:10.1021/nl0727157. PMID 18095738. http://www.stanford.edu/group/cui_group/papers/High%20Capacity%20Li-ion%20Battery%20Anodes%20Using%20Ge%20Nanowires.pdf.
- ^ Wenige, Niemann, et al. (30 May 1998). Liquid Electrolyte Systems for Advanced Lithium Batteries (PDF). cheric.org; Chemical Engineering Research Information Center(KR). Retrieved 11 June 2010.
- ^ Balbuena, P.B., Wang, Y.X., eds. Lithium Ion Batteries: Solid Electrolyte Interphase 2004 Imperial College Press, London
- ^ Syzdek, Jaroslaw, et al., Journal of Power Sources, 173, 2007, p712-720 doi:10.1016/j.jpowsour.2007.05.061
- ^ Syzdek, Jaroslaw, et al., Electrochimica Acta, 55, 2010, p1314-1322, doi:10.1016/j.electacta.2009.04.025
- ^ Syzdek, Jaroslaw, et al., Journal of Power Sources, 194, 2009, p66-72, doi:10.1016/j.jpowsour.2009.01.070
- ^ Winter & Brodd 2004, pp. 4256, 4258
- ^ , Winter & Brodd 2004, p. 4254
- ^ a b c Winter & Brodd 2004, p. 4259
- ^ name="J. Brodd, Chem 2004">Winter & Brodd 2004, p. 4258
- ^ Altair Nano: Power & Energy Systems
- ^ "A123 M1 cell specifications". http://www.a123systems.com/cms/product/pdf/1/_ANR26650M1A.pdf.
- ^ Winter & Brodd 2004, p. 4258
- ^ Battery Management Systems for Large Lithium-Ion Battery Packs page 12
- ^ Buchmann, Isidor (200804). "Choosing a battery that will last". Isidor Buchmann (CEO of Cadex Electronics Inc.). http://www.buchmann.ca/Article9-Page1.asp.
- ^ Battery Management Systems for Large Lithium-Ion Battery Packs page 229
- ^ Buchmann, Isidor (September 2006). "BatteryUniversity.com: How to prolong lithium-based batteries". Cadex Electronics Inc.. http://www.batteryuniversity.com/parttwo-34.htm.
- ^ Spotnitz, R.; Franklin, J. (2003). "Abuse behavior of high-power, lithium-ion cells". Journal of Power Sources (Elsevier) 113: 81–100. doi:10.1016/S0378-7753(02)00488-3.
- ^ Buchmann, Isidor (February 2003). "Advanced battery analyzers". Isidor Buchmann. http://www.batteryuniversity.com/parttwo-43.htm. Retrieved 26 December 2009.
- ^ "Lithium-ion Battery Charging Basics". PowerStream Technologies. http://www.powerstream.com/li.htm. Retrieved 4 December 2010.
- ^ AeroVironment achieves electric vehicle fast-charge milestone avinc.com; AeroVironment, 30 May 2007. (Press release). "Test rapidly recharges a battery pack designed for use in passenger vehicles. 10-minute recharge restores enough energy to run electric vehicle for two hours at 60 miles per hour."
- ^ "The 3 charging stages". Liionbms.com. http://liionbms.com/php/wp_charging_stages.php. Retrieved 8 October 2011.
- ^ Battery Management Systems for Large Lithium Ion Battery Packs section 6.2.3
- ^ Kevin Jost [ed.] (October 2006). Tech Briefs: CPI takes new direction on Li-ion batteries (PDF). aeionline.org; Automotive Engineering Online. Archived from the original. Retrieved 11 June 2010.
- ^ Voelcker, John (September 2007). Lithium Batteries Take to the Road. IEEE Spectrum. Retrieved 15 June 2010.
- ^ Loveday, Eric (23 April 2010). "Hitachi develops new manganese cathode, could double life of li-ion batteries". http://green.autoblog.com/2010/04/23/hitachi-develops-new-manganese-cathode-could-double-life-of-li/. Retrieved 11 June 2010..
- ^ Nikkei (29 November 2009). Report: Nissan On Track with Nickel Manganese Cobalt Li-ion Cell for Deployment in 2015 Green Car Congress (blog). Retrieved 11 June 2010.
- ^ Elder, Robert and Zehr, Dan (16 February 2006). Valence sued over UT patent Austin American-Statesman (courtesy Bickle & Brewer Law Firm). Archived from the original. Retrieved 11 June 2010.
- ^ Bulkeley, William M. (26 November 2005). "New Type of Battery Offers Voltage Aplenty, at a Premium". The Day: p. E6. http://news.google.com/newspapers?id=fCAiAAAAIBAJ&pg=1148,5896335.
- ^ A123Systems (2 November 2005). A123Systems Launches New Higher-Power, Faster Recharging Li-Ion Battery Systems Green Car Congress; A123Systems (Press release). Retrieved 11 May 2010.
- ^ "Imara Corporation website". Imaracorp.com. http://www.imaracorp.com. Retrieved 8 October 2011.
- ^ O'Dell, John (17 December 2008). Fledgling Battery Company Says Its Technology Boosts Hybrid Battery Performance Green Car Advisor; Edmunds Inc. Retrieved 11 June 2010.
- ^ B. L. Ellis, W. R. M. Makahnouk, Y. Makimura, K. Toghill & L. F. Nazar (9 September 2007). "A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries". Nature Materials (Nature Materials) 6 (10): 749–753. Bibcode 2007NatMa...6..749E. doi:10.1038/nmat2007. PMID 17828278. http://www.nature.com/nmat/journal/v6/n10/abs/nmat2007.html. Retrieved 8 October 2009.
- ^ a b "A Research First: Lithium Air Battery Development (Press Release)". 17 November 2009. http://www-ig.udayton.edu/News/Article/?contentId=25610. Retrieved 11 June 2010.
- ^ Jian Hong, C. S. Wang, Shailesh Upreti and M. Stanley Whittinghama. "Vanadium Modified LiFePO4 Cathode for Li-ion Batteries". ECS (ETS) 12 (2): A33. http://link.aip.org/link/?ESLEF6/12/A33/1. Retrieved 11 June 2010.
- ^ "... Acceptance of the First Grid-Scale, Battery Energy Storage System" (Press release). Altair Nanotechnologies. 21 November 2008. http://www.b2i.us/profiles/investor/ResLibraryView.asp?ResLibraryID=27574&GoTopage=1&BzID=546&Category=1183&a=. Retrieved 8 October 2009.
- ^ Marty Ozols (11 November 2009). Altair Nanotechnologies Power Partner - The Military Systemagicmotives (personal webpage). Retrieved 11 June 2010.
- ^ "Proterra Corporate website". Proterra. http://www.proterraonline.com/partners.asp. Retrieved 8 October 2009.
- ^ "Microsoft PowerPoint - 061125 Altair EDTA Presentation". Altairnano.com. http://www.altairnano.com/documents/AltairnanoEDTAPresentation.pdf. Retrieved 8 October 2011.
- ^ Blain, Loz (2 November 2007). "Subaru doubles the battery range on its electric car concept". gizmag. http://www.gizmag.com/go/8281/. Retrieved 8 October 2009.
- ^ "Li-Ion Rechargeable Batteries Made Safer". Nikkei Electronics Asia. 29 January 2008. http://techon.nikkeibp.co.jp/article/HONSHI/20080129/146549/. Retrieved 8 October 2009.
- ^ Ki Tae Nam, Dong-Wan Kim, et. al. (6 April 2006). Virus-Enabled Synthesis and Assembly of Nanowires for Lithium Ion Battery Electrodes. Science Express (preprint). Retrieved 11 June 2010.(subscription required)
- ^ "gatech.edu". http://www.mse.gatech.edu.
- ^ Kara Evanoff, Alexandre Magasinskiy, Junbing Yang and Gleb Yushin (21 April 2011). "Nanosilicon-Coated Graphene Granules as Anodes for Li-Ion Batteries". Advanced Energy Materials 1 (4): 495. doi:10.1002/aenm.201100071.
- ^ Palca, Joe (6 April 2009). Hidden Ingredient In New, Greener Battery: A Virus. npr.org; National Public Radio. Retrieved 11 June 2010.
- ^ Zandonella, Catherine (11 April 2009). "Battery grown from "armour plated" viruses". New Scientist (Tribune media Services International) 202 (2703): 1. http://www.newscientist.com/article/mg20227035.400-batteries-grown-from-armourplated-viruses.html.
- ^ Bullis, Kevin (28 September 2006). "Powerful Batteries That Assemble Themselves". technologyreview.com. Technology Review. http://www.technologyreview.com/energy/17553/?a=f. Retrieved 15 June 2010.
- ^ "Bad Virus Put to Good Use". Clark School of Engineering, University of Maryland. 6 December 2010. http://www.eng.umd.edu/news/news_story.php?id=5374. Retrieved December, 2010.
- ^ "Self-Assembled Nanocomposites Boost Lithium-Ion Battery Anodes". Nature Materials and Georgia Institute of Technology. 15 March 2010. http://www.gatech.edu/newsroom/release.html?nid=54920. Retrieved March, 2010.
- ^ "New Nanowire Battery Holds 10 Times The Charge Of Existing Ones". sciencedaily.com. Science Daily. 20 December 2007. http://www.sciencedaily.com/releases/2007/12/071219103105.htm.
- ^ Dennis, Lyle (21 December 2007). "Interview with Dr. Cui, Inventor of Silicon Nanowire Lithium-ion Battery Breakthrough". GM-Volt. http://www.gm-volt.com/2007/12/21/gm-voltcom-interview-with-dr-cui-inventor-of-silicon-nanowire-lithium-ion-battery-breakthrough. Retrieved 8 October 2009.
- ^ Y. Oumellal, A. Rougier, G. A. Nazri, J-M. Tarascon & L. Aymard (12 October 2008). "Metal hydrides for lithium-ion batteries". Nature Materials 7 (11): 916–921. Bibcode 2008NatMa...7..916O. doi:10.1038/nmat2288. PMID 18849978. http://www.nature.com/nmat/journal/v7/n11/abs/nmat2288.html. Retrieved 8 October 2009.
- ^ "Laboratory of the Prof. Gleb Yushin". Nano-tech.gatech.edu. 31 August 2011. http://www.nano-tech.gatech.edu/. Retrieved 8 October 2011.
- ^ Benjamin Hertzberg, Alexander Alexeev and Gleb Yushin (8 June 2010). "Deformations in Si−Li Anodes Upon Electrochemical Alloying in Nano-Confined Space". Journal of the American Chemical Society 132 (25): 8548–8549. doi:10.1021/ja1031997. PMID 20527882. http://pubs.acs.org/doi/abs/10.1021/ja1031997. Retrieved 8 June 2010.
- ^ Bourzac, Katherine (30 September 2011). "A Simple Way to Boost Battery Storage". Technology Review. http://www.technologyreview.com/energy/38732/.
- ^ Welcome to Ener1. Ener1 (Press release). Archived from the original 8 July 2006. Retrieved 11 June 2010.
- ^ EnerDel Technical Presentation (PDF). EnerDel Corporation. 29 October 2007. Archived from the original. Retrieved 11 June 2010.
- ^ Bullis, Kevin (22 June 2006). Higher-Capacity Lithium-Ion Batteries Technology Review. Retrieved 11 June 2010.
- ^ a b c "How to Prolong Lithium-based Batteries". Battery University. http://batteryuniversity.com/parttwo-34.htm. Retrieved 8 October 2011.
- ^ L.M. Cristo, T. B. Atwater. Characteristics and Behavior of 1M LiPF6 1EC:1DMC Electrolyte at Low Temperatures. Fort Monmouth, NJ: U.S. Army Research.
- ^ "Modelling capacity fade in Lithium-ion cells, Bor Yann Liaw, Jungst, Nagasubramanian, and Doughty, Sandia National Laborator - Analysis of Lithium-Ion Battery Degradation during Thermal Aging" (PDF). http://www.electrochem.org/dl/ma/204/pdfs/0253.PDF. Retrieved 8 October 2011.
- ^ Andrea, Davide (21 August 2008). About Battery Management Systems. ELithion LLC. Retrieved 15 June 2010.
- ^ Andrea, Davide (19 September 2008). White Paper - CCCV chargers: a false sense of security. ELithion LLC. Retrieved 15 June 2010.
- ^ Electrochem Commercial Power (9 September 2006). "Safety and handling guidelines for Electrochem Lithium Batteries" (PDF). http://marine.rutgers.edu. Rutgers University. http://marine.rutgers.edu/~haldeman/Instruments/lithium_safety/Electrochem_Lithium_safety_15-SAF-0043.pdf. Retrieved 21 May 2009.
- ^ Cringely, Robert X. (1 September 2006). "Safety Last". The New York Times. http://www.nytimes.com/2006/09/01/opinion/01cringely.html. Retrieved 14 April 2010.
- ^ Nokia issues BL-5C battery warning, offers replacement. Wikinews. 14 August 2007. http://en.wikinews.org/wiki/Nokia_issues_BL-5C_battery_warning%2C_offers_replacement. Retrieved 8 October 2009.
- ^ Staff (27 July 2007). Nokia N91 cell phone explodes Mukamo - Filipino News (blog). Retrieved 15 June 2010.
- ^ Tullo, Alex. (21 August 2006). "Dell Recalls Lithium Batteries". Chemical and Engineering News:11; American Chemical Society. Retrieved 15 June 2010.
- ^ Hales, Paul (21 June 2006). Dell laptop explodes at Japanese conference. The Inquirer. Retrieved 15 June 2010.
- ^ "Kyocera Launches Precautionary Battery Recall, Pursues Supplier of Counterfeit Batteries" (Press release). Kyocera Wireless. 28 October 2004. Archived from the original on 7 January 2006. http://web.archive.org/web/20060107210116/http://www.kyocera-wireless.com/news/20041028_2.htm. Retrieved 15 June 2010.
- ^ a b "Safe Travel". Safetravel.dot.gov. U.S. Department of Transportation. 1 January 2008. http://safetravel.dot.gov/whats_new_batteries.html. Retrieved 8 October 2009.
- ^ Galbraith, Rob (3 January 2008). "U.S. Department of Transportation revises lithium battery rules press release". Little Guy Media. http://www.robgalbraith.com/bins/content_page.asp?cid=7-9206-9211. Retrieved 11 May 2009.
- ^ Prohibitions - 6.3.12 - Dangerous, offensive and indecent articles (PDF). Hong Kong Post Office Guide. December 2009. Retrieved 15 June 2010.
- ^ International Mail > FAQs > Goods/Services: Shipping a Laptop Japan Post Service Co. Ltd. Retrieved 15 June 2010.
- ^ http://arstechnica.com/science/news/2011/08/new-solid-state-compound-beats-old-school-lithium-ion-batteries.ars. Retrieved 3 November 2011.
- ^ a b Melody Voth (6 December 2010). "Battery Booster". http://pubs.acs.org/cen/email/html/8849bus1.html. Retrieved 9 February 2011.
References
- Winter, M.; Brodd, J. (2004). "What Are Batteries, Fuel Cells, and Supercapacitors?" (PDF). Chemical Review 104 (104): 4245. doi:10.1021/cr020730k. http://pubs.acs.org/doi/pdf/10.1021/cr020730k. Retrieved 25 July 2010.
External links
- Asahi Kasei Corporation -- Father of the lithium-ion battery
- Lithium batteries at the Open Directory Project
- Argonne opens chapter in battery research -- lithium air. Argonne National Labs. Press release. 14 September 2009.
- Battery and Energy Technologies - Rechargeable Lithium batteries. Electropaedia; Woodbank Communications Ltd. Updated 28 April 2010.
- Stanford's nanowire battery holds 10 times the charge of existing ones. Stanford Report, 18 December 2007. Press release.
- The Lithium Ion Battery. E-Articles.com. (Self-publishing site).
- The Future of Electric Vehicles: Setting the Record Straight on Lithium Availability. Journal of Energy Security, 27 August 2009. Keith Evans.
- Researchers from Spheric Technologies and Arizona State University Describe Major Advances in the Use of Microwaves to Produce Key Lithium Ion Battery Materials; Present Papers at MS&T'10 Conference, 17-21 October. Spheric Technologies. Press release. 18 October 2010.